
In clean rooms and laboratories, air purity isn’t just about comfort—it’s about data integrity, product quality, regulatory compliance, and safety. Even microscopic contamination can invalidate months of research, compromise product batches worth millions, or create safety hazards that shut down operations.
Professional-grade air purification systems designed for controlled environments ensure your critical work proceeds without contamination interference.
Understanding Clean Room and Laboratory Air Quality Standards
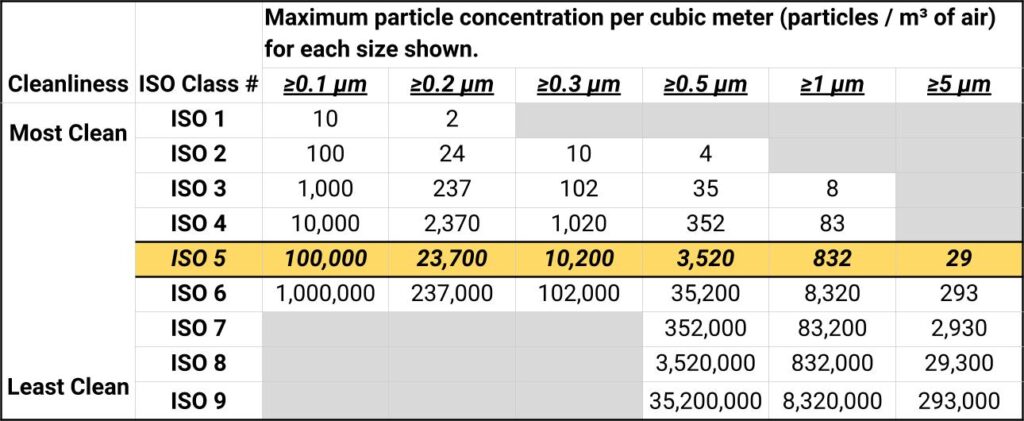
ISO 14644 Clean Room Classifications
Clean rooms are classified by the maximum allowable particle concentration per cubic meter:
ISO Class 1 (Ultra-Clean):
- ≤10 particles (≥0.1 μm) per cubic meter
- Semiconductor manufacturing, nanotechnology
- Requires ULPA filtration (99.999% efficiency)
ISO Class 3 (Sterile Manufacturing):
- ≤1,000 particles (≥0.1 μm) per cubic meter
- Pharmaceutical sterile manufacturing
- Medical device assembly
ISO Class 5 (Pharmaceutical):
- ≤100,000 particles (≥0.1 μm) per cubic meter
- Aseptic processing areas
- Critical pharmaceutical operations
ISO Class 7 (General Laboratory):
- ≤10,000,000 particles (≥0.1 μm) per cubic meter
- Research laboratories, quality control
- Non-sterile pharmaceutical manufacturing
Laboratory-Specific Standards
Research Laboratories:
- ASHRAE 62.1 ventilation standards
- NIH guidelines for biological research
- OSHA laboratory safety requirements
- Institution-specific protocols
Clinical Laboratories:
- CLIA (Clinical Laboratory Improvement Amendments)
- CAP (College of American Pathologists) standards
- ISO 15189 medical laboratory requirements
- State health department regulations
Specialized Applications:
- FDA cGMP for pharmaceutical research
- EPA guidelines for environmental testing
- USDA standards for food safety testing
- DEA requirements for controlled substance research
Explore our comprehensive Clean Room Compliance Solutions Guide
Critical Contamination Sources in Controlled Environments
Personnel-Generated Contamination
Humans are the largest source of contamination in clean environments:
Particle Generation:
- 100,000+ particles per minute from normal movement
- Skin cells, hair, and textile fibers
- Respiratory droplets and moisture
- Cosmetics and personal care product residues
Mitigation Strategies:
- Strategic air purifier placement near personnel entry
- Positive pressure maintenance
- Continuous air circulation and filtration
- Real-time particle monitoring
Equipment and Process Contamination
Laboratory equipment and processes create specific contamination challenges:
Chemical Vapors:
- Solvent evaporation from analytical instruments
- Reagent off-gassing and reactions
- Cleaning agent residues
- Sample preparation emissions
Particulate Generation:
- Instrument mechanical operation
- Sample handling and processing
- Packaging material particles
- Equipment wear and degradation
External Contamination Sources
Outside influences can compromise controlled environments:
Infiltration Points:
- HVAC system contamination
- Door and window sealing failures
- Construction and maintenance activities
- Outdoor pollution during peak hours
Advanced Air Purification Technologies for Critical Environments
Ultra-Low Penetration Air (ULPA) Filtration
For the most demanding applications requiring maximum particle removal:
ULPA Performance:
- 99.999% efficiency at 0.12 microns
- Superior to HEPA for submicron particles
- Essential for semiconductor and nanotechnology
- Validated performance testing available
Applications:
- ISO Class 1-3 clean rooms
- Electron microscopy facilities
- Precision manufacturing environments
- Critical research applications
High-Efficiency Gas-Phase Filtration
Specialized carbon and chemical filtration for laboratory air purification:
Advanced Media Types:
- Impregnated activated carbon for acid gases
- Molecular sieves for specific chemicals
- Potassium permanganate for oxidizable compounds
- Custom blends for unique applications
Chemical Removal Capabilities:
- Organic solvents and vapors
- Acid and base gases
- Mercury vapor in analytical labs
- Formaldehyde and glutaraldehyde
- Specialized research chemicals
HEPA Plus Technology
Enhanced HEPA systems combining particle and gas-phase removal:
Integrated Features:
- True HEPA particle filtration
- Activated carbon VOC removal
- UV-C sterilization capability
- Real-time monitoring and control
- Validated performance documentation
Laminar Flow and Unidirectional Airflow
Creating contamination-free work zones within larger spaces:
Laminar Flow Benefits:
- Particle-free workspace creation
- Protection of sensitive samples
- Operator breathing zone protection
- Critical process area isolation
Laboratory-Specific Air Purification Applications
Analytical Laboratories
Chemical analysis requires contamination-free environments and operator protection:
Critical Requirements:
- Removal of interfering compounds
- Protection from toxic vapors
- Maintenance of analytical sensitivity
- Compliance with method requirements
- Sample integrity preservation
Recommended Systems:
- High-capacity gas-phase filtration
- Real-time chemical monitoring
- Emergency purge capabilities
- Redundant filtration stages
Biological Research Facilities
Life science research demands sterile conditions and containment:
Biosafety Considerations:
- Pathogen containment and removal
- Cross-contamination prevention
- Aerosol capture from biological processes
- Compliance with biosafety level requirements
Specialized Features:
- HEPA filtration with UV-C backup
- Positive/negative pressure control
- Emergency containment protocols
- Validated sterilization cycles
Pharmaceutical Research and Development
Drug development requires the highest standards of environmental control:
GMP Requirements:
- Validated cleaning procedures
- Contamination prevention protocols
- Environmental monitoring programs
- Change control documentation
- Qualification and validation testing
System Capabilities:
- Ultra-high efficiency filtration
- Continuous monitoring and alarming
- Data logging and trending
- Qualification documentation packages
Materials Science and Nanotechnology
Precision manufacturing and research require particle-free environments:
Ultra-Clean Requirements:
- Sub-micron particle control
- Static elimination capabilities
- Chemical purity maintenance
- Process interference prevention
Explore our Laboratory Air Purification Product Guide
Clean Room Design and Integration Considerations
Airflow Pattern Design
Proper airflow ensures contamination removal without creating turbulence:
Unidirectional Flow:
- Top-to-bottom laminar flow
- Minimal turbulence generation
- Contamination sweep-out patterns
- Work surface protection
Mixed Flow Systems:
- Strategic supply and exhaust placement
- Contamination dilution strategies
- Energy efficiency optimization
- Multi-zone control capabilities
Pressure Differential Management
Maintaining proper pressure relationships prevents contamination migration:
Pressure Cascade Design:
- Highest pressure in cleanest areas
- Graduated pressure zones
- Containment area negative pressure
- Emergency pressure control
Integration with Building Systems
HVAC Coordination:
- Primary air handling integration
- Secondary treatment systems
- Energy recovery optimization
- Maintenance scheduling coordination
Environmental Controls:
- Temperature and humidity coordination
- Lighting and electrical integration
- Security and access control
- Emergency shutdown procedures
Performance Validation and Testing
Commissioning and Qualification
Installation Qualification (IQ):
- Equipment specification verification
- Installation documentation review
- Utility connection validation
- Safety system verification
Operational Qualification (OQ):
- Performance parameter testing
- Alarm and safety function verification
- Operating range confirmation
- Control system validation
Performance Qualification (PQ):
- Particle count verification
- Chemical removal efficiency testing
- Airflow pattern validation
- Environmental condition maintenance
Ongoing Performance Monitoring
Continuous Monitoring Systems:
- Real-time particle counting
- Chemical concentration measurement
- Pressure differential tracking
- System performance trending
- Automated alarm and notification
Regular Testing Protocols:
- Daily operational checks
- Weekly performance verification
- Monthly comprehensive testing
- Quarterly validation reviews
- Annual re-certification procedures
Maintenance and Service for Critical Environments
Preventive Maintenance Programs
Filter Management:
- Predictive replacement scheduling
- Pre-filter protection strategies
- Filter integrity testing procedures
- Disposal and documentation protocols
System Optimization:
- Performance trending analysis
- Energy efficiency monitoring
- Predictive maintenance scheduling
- Component lifecycle management
Emergency Response and Support
24/7 Support Services:
- Emergency repair response
- Temporary filtration solutions
- Contamination event protocols
- System recovery procedures
Spare Parts and Inventory:
- Critical component availability
- Local service technician network
- Rapid response capabilities
- Documentation and traceability
Compliance Documentation
Validation Maintenance:
- Performance data collection
- Trend analysis and reporting
- Deviation investigation procedures
- Continuous compliance verification
Cost Considerations and ROI for Critical Environments
Initial Investment Analysis
System Costs:
- Equipment purchase or lease options
- Installation and commissioning expenses
- Validation and documentation costs
- Training and certification requirements
Hidden Cost Factors:
- Energy consumption optimization
- Maintenance and service agreements
- Filter replacement programs
- Compliance documentation requirements
Value Protection and ROI
Research Protection:
- Prevented data loss from contamination
- Reduced experimental repeat costs
- Accelerated research timelines
- Enhanced publication credibility
Manufacturing Benefits:
- Improved product yield rates
- Reduced batch rejection costs
- Enhanced product quality consistency
- Regulatory compliance assurance
Risk Mitigation:
- Avoided regulatory penalties
- Reduced liability exposure
- Insurance premium reductions
- Reputation protection value
Total Cost of Ownership
Lifecycle Analysis:
- Equipment depreciation schedules
- Operating cost projections
- Maintenance expense planning
- Upgrade and expansion considerations
Selecting Your Clean Room Air Purification Partner
Critical Evaluation Criteria
Technical Expertise:
- Clean room design experience
- Regulatory compliance knowledge
- Validation and documentation capabilities
- Industry-specific application understanding
Service and Support:
- Local service technician availability
- Emergency response capabilities
- Spare parts inventory management
- Ongoing training and education
Quality Assurance:
- ISO certification and quality systems
- Validation protocol development
- Performance guarantee programs
- Continuous improvement processes
Questions to Ask Potential Suppliers
Technical Capabilities:
- What clean room classes have you designed for?
- How do you ensure regulatory compliance?
- What validation documentation do you provide?
- How do you handle emergency situations?
Service and Support:
- What’s your local service response time?
- How do you manage spare parts inventory?
- What training do you provide our staff?
- How do you stay current with regulations?
Industry-Specific Applications
Pharmaceutical Manufacturing
cGMP Compliance Requirements:
- Validated cleaning procedures
- Environmental monitoring programs
- Change control documentation
- Deviation investigation protocols
Critical Applications:
- Sterile product manufacturing
- Active pharmaceutical ingredient processing
- Packaging and labeling operations
- Quality control laboratories
Biotechnology Research
Biosafety and Containment:
- BSL-1 through BSL-4 requirements
- Pathogen containment protocols
- Aerosol capture and treatment
- Emergency response procedures
Semiconductor Manufacturing
Ultra-Clean Requirements:
- Sub-micron particle elimination
- Chemical purity maintenance
- Static control integration
- Process interference prevention
Medical Device Manufacturing
FDA Quality System Requirements:
- Design control compliance
- Risk management protocols
- Validation and verification procedures
- Post-market surveillance support
Getting Started: Your Clean Room Air Purification Journey
Implementing air purification in controlled environments requires specialized expertise and careful planning. Our clean room specialists understand the unique challenges of maintaining critical environments while meeting stringent regulatory requirements.
Immediate Next Steps:
- Environmental Assessment
- Current air quality evaluation
- Contamination source identification
- Regulatory requirement review
- Existing system analysis
- Solution Design
- Custom system specification
- Integration planning
- Validation protocol development
- Installation timeline creation
- Implementation and Validation
- Professional installation
- Comprehensive testing and qualification
- Staff training and certification
- Documentation package delivery
Ready to Protect Your Critical Environment?
The clean room air purification experts at Airgle are ready to design a solution that meets your exact specifications while ensuring full regulatory compliance.
Schedule Your Clean Room Air Quality Consultation
Get expert guidance on maintaining your critical environment with confidence.
✓ Regulatory compliance expertise ✓ Custom solution design ✓ Complete validation documentation ✓ Ongoing technical support
Compare laboratory air purification systems or explore our complete commercial air purification guide